Structural and Dynamical Insight into PPARγ Antagonism: In silico Study of the Ligand-Receptor Interactions of non-Covalent Antagonists
Filip Fratev *, Ivanka Tsakovska , Merilin Al Sharif , Elina Mihaylova and Ilza Pajeva
Micar21 Ltd., Persenk Str. 34B, 1407 Sofia, Bulgaria;
International Journal of Molecular Sciences
Abstract
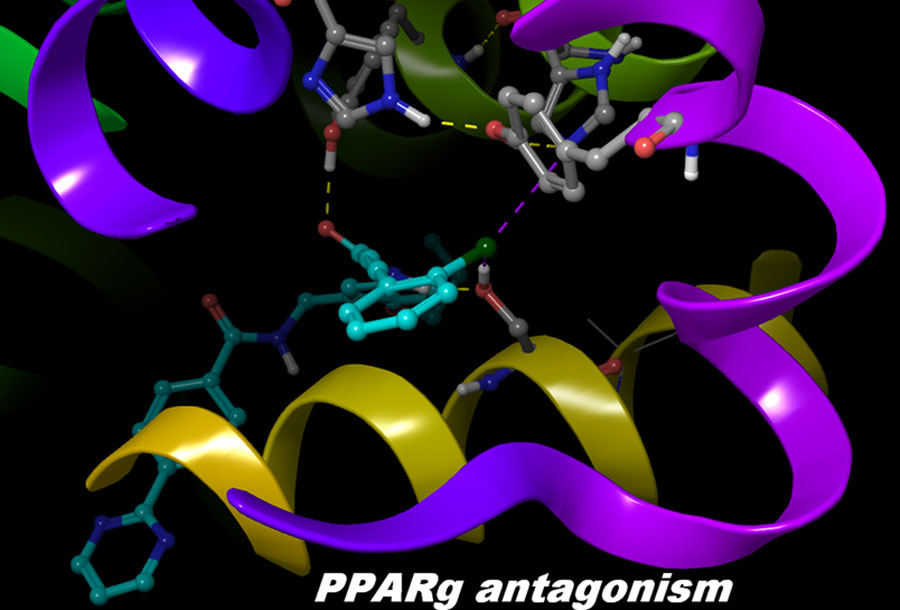
The structural and dynamical properties of the PPARγ nuclear receptor have been broadly studied in its agonist state but little is known about the key features required for the receptor antagonistic activity. Here we report a series of molecular dynamics (MD) simulations in combination with free energy estimation of the recently discovered class of non-covalent PPARγ antagonists. Their binding modes and dynamical behavior are described in details. Two key interactions have been detected within the cavity between helices H3, H11 and the activation helix H12, as well as with H12. The strength of the ligand-amino acid residues interactions has been analyzed in relation to the specificity of the ligand dynamical and antagonistic features. According to our results, the PPARγ activation helix does not undergo dramatic conformational changes, as seen in other nuclear receptors, but rather perturbations that happen through a significant ligand-induced reshaping of the ligand-receptor and the receptor-coactivator binding pockets. The H12 residue Tyr473 and the charge clamp residue Glu471 play a central role for the receptor transformations. Our results also demonstrate that MD can be a helpful tool for the compound phenotype characterization (full agonists, partial agonists or antagonists) when insufficient experimental data are available.
