Discovery of a Novel Selective PPARγ Ligand with Partial Agonist Binding Properties by Integrated in Silico/in Vitro Work Flow
Irene Kouskoumvekaki *†, Rasmus K. Petersen ‡§, Filip Fratev †∥, Olivier Taboureau †⊥, Thomas E. Nielsen # ∇, Tudor I. Oprea †○, Si B. Sonne §, Esben N. Flindt ‡§, Svava Ósk Jónsdóttir †◆, and Karsten Kristiansen *§
† Center for Biological Sequence Analysis, Department of Systems Biology, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark
‡ BioLigands, Science Park, 5230, Odense, Denmark
§ Department of Biology, University of Copenhagen, Ole Maaløes Vej 5, DK-2200, Copenhagen, Denmark
∥ Micar 21 Ltd., 34B Persenk Str., 1407, Sofia, Bulgaria
⊥ UMR-S973, MTi, University Paris Diderot, F-75013 Paris, France
# Department of Chemistry, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark
∇ Singapore Centre on Environmental Life Sciences Engineering, Nanyang Technological University, Singapore 637551
○ Translational Informatics Division, Department of Internal Medicine, MSC09 5025, University of New Mexico School of Medicine, Albuquerque, New Mexico 87131, United States
◆ Department of Toxicology and Risk Assessment, National Food Institute, Technical University of Denmark, Mørkhøj Bygade 19, DK-2860 Søborg, Denmark
J. Chem. Inf. Model., 2013, 53 (4), pp 923–937
DOI: 10.1021/ci3006148
Publication Date (Web): February 24, 2013
Copyright © 2013 American Chemical Society
Section:Pharmacology
Abstract
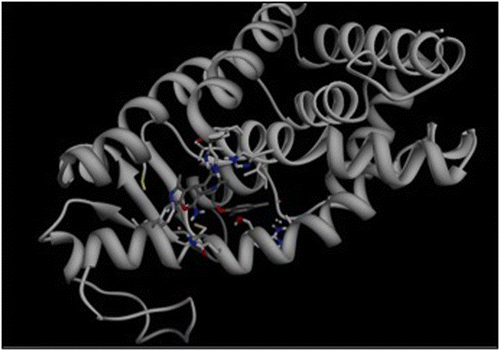
Full agonists to the peroxisome proliferator-activated receptor (PPAR)γ, such as Rosiglitazone, have been associated with a series of undesired side effects, such as weight gain, fluid retention, cardiac hypertrophy, and hepatotoxicity. Nevertheless, PPARγ is involved in the expression of genes that control glucose and lipid metabolism and is an important target for drugs against type 2 diabetes, dyslipidemia, atherosclerosis, and cardiovascular disease. In an effort to identify novel PPARγ ligands with an improved pharmacological profile, emphasis has shifted to selective ligands with partial agonist binding properties. Toward this end we applied an integrated in silico/in vitro workflow, based on pharmacophore- and structure-based virtual screening of the ZINC library, coupled with competitive binding and transactivation assays, and adipocyte differentiation and gene expression studies. Hit compound 9 was identified as the most potent ligand (IC50 = 0.3 μM) and a relatively poor inducer of adipocyte differentiation. The binding mode of compound 9 was confirmed by molecular dynamics simulation, and the calculated free energy of binding was −8.4 kcal/mol. A novel functional group, the carbonitrile group, was identified to be a key substituent in the ligand–protein interactions. Further studies on the transcriptional regulation properties of compound 9 revealed a gene regulatory profile that was to a large extent unique, however functionally closer to that of a partial agonist.
Proteins: Structure, Function, and Bioinformatics (Full version)
